Abstract
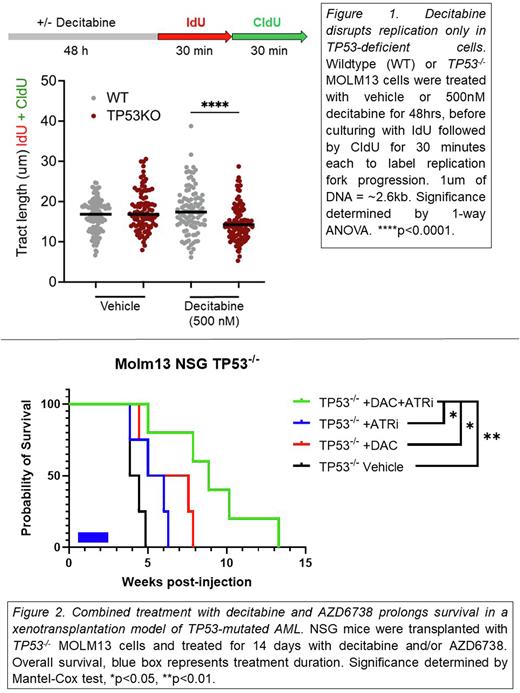
Mutations of TP53 are found in approximately 10% of cases of de novo AML/MDS and approximately 30% of therapy related AML/MDS. These patients have an exceptionally poor prognosis, with median survival of 6-9 months. Many patients with TP53-mutant AML/MDS respond to hypomethylating agents, although the response is not durable, similar to standard induction therapy. The mechanisms that contribute to the relative sensitivity of TP53-mutated AML/MDS to hypomethylating agents is unknown. Hypomethylating agents work, at least in part, by inhibiting DNMT1, the major DNA methyltransferase responsible for maintaining methylation of genomic DNA. Loss of DNMT1 and DNA hypomethylation has been previously shown to induce DNA replication stress and activate the ATM-Rad3-related (ATR) pathway. TP53 has multiple roles in the replication stress response, including control of the G1/S checkpoint and regulation of DNA replication progression and restart. Based on these observations, we hypothesize that ATR inhibition selectively sensitizes TP53-mutated MDS/AML to decitabine-induced replication stress. To test this hypothesis, initial studies focused on MOLM13 cells, a TP53-wildtype human AML cell line. Treatment with decitabine for 4 days induced global hypomethylation, and increased phosphorylation of CHK1, which is indicative of ATR activation. We next performed single-molecule DNA fiber assays to assess replication fork progression after exposure to decitabine. We used CRISPR-Cas9 gene editing to generate TP53-/- (or control) MOLM13 cells. In untreated cells, no difference in replication fork progression rate was observed between wildtype and TP53-/- MOLM13 cells (Figure 1). However, after decitabine treatment, a significant decrease in replication fork length was observed in TP53-/- MOLM13, but not wildtype MOLM13 cells. These data show that p53 is required for the efficient resolution of replication stress induced by decitabine. Since ATR is a major regulator of replication stress, we next examined the response of TP53-/- MOLM13 cells to decitabine alone, AZD6738 (an ATR inhibitor) alone, or the combination. Compared to controls, TP53-/- MOLM13 cells showed a modest increased sensitivity to decitabine, but not AZD6738. However, strong synergistic killing was observed with the combination in TP53-/-, but not control, MOLM13 cells (Zip synergy score of 10.1 vs 5.8). Similar results were observed in a second cell line, murine AML1. Cell cycle analysis showed that combined treatment results in a selective increase in polyploid TP53-/- MOLM13 cells, which is suggestive of mitotic catastrophe.
We next developed a xenotransplantation model of TP53-mutated AML by transplanting TP53-/- or control MOLM13 cells into NSG mice. After 4 days to allow for engraftment, mice were treated with vehicle alone, decitabine alone (0.1mg/kg), AZD6738 alone (50mg/kg), or the combination for 14 days. In mice engrafted with wildtype MOLM13 cells, no significant benefit of single agent or combination therapy was observed. In contrast, treatment with decitabine alone modestly, but significantly, prolonged survival in mice engrafted with TP53-/- MOLM13 cells (Figure 2). Importantly, the survival of mice treated with combined AZD6738 and decitabine had a significantly prolonged survival compared to decitabine alone. These data show that even a single cycle of AZD6738 and decitabine is sufficient to prolong survival in the xenograft model of human AML. Finally, to assess the impact of combination treatment on TP53-mutant hematopoietic stem cells (HSCs), we established mixed bone marrow chimeras transplanted with both wildtype and R172H TP53 bone marrow. Mice were treated with either cis-platinum or decitabine ± AZD6738. Whereas cis-platinum treatment resulted in a significant expansion of TP53-mutant HSCs, treatment with decitabine plus AZD6738 selectively inhibited TP53-mutant HSCs. Collectively, these data support the hypothesis that TP53-mutated AML/MDS is selectively sensitive to ATR inhibition in combination with decitabine-induced replicative stress. A clinical trial of this drug combination in patients with TP53-mutated AML/MDS is being developed.
Disclosures
Link:Roche: Other: Roche provided Idasanutlin free of charge. No compensation was provided, and Roche was not involved in design, conduction, or analysis of experiments..
Author notes
Asterisk with author names denotes non-ASH members.